Heat Convection
As they collide, the faster molecules give up some of their energy to the slower molecules. The slower molecules gain more thermal energy and collide with other molecules in the cooler object. This process continues until heat energy from the warmer object spreads throughout the cooler object. Some substances conduct heat more easily than others.
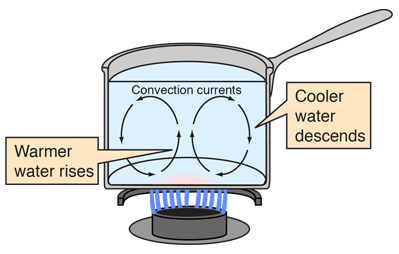
Solids are better conductor than liquids and liquids are better conductor than gases. Metals are very good conductors of heat, while air is very poor conductor of heat. You experience heat transfer by conduction whenever you touch something that is hotter or colder than your skin e. In liquids and gases, convection is usually the most efficient way to transfer heat. Convection occurs when warmer areas of a liquid or gas rise to cooler areas in the liquid or gas. As this happens, cooler liquid or gas takes the place of the warmer areas which have risen higher.
This cycle results in a continous circulation pattern and heat is transfered to cooler areas. You see convection when you boil water in a pan. The bubbles of water that rise are the hotter parts of the water rising to the cooler area of water at the top of the pan. You have probably heard the expression "Hot air rises and cool air falls to take its place" - this is a description of convection in our atmosphere. Because the molecules in aggregate retain their random motion, the total heat transfer is then due to the superposition of energy transport by random motion of the molecules and by the bulk motion of the fluid.
It is customary to use the term convection when referring to this cumulative transport and the term advection when referring to the transport due to bulk fluid motion. In many real-life applications e. Internal and external flow can also classify convection. Internal flow occurs when a fluid is enclosed by a solid boundary such when flowing through a pipe. An external flow occurs when a fluid extends indefinitely without encountering a solid surface. Both of these types of convection, either natural or forced, can be internal or external because they are independent of each other.
Further classification can be made depending on the smoothness and undulations of the solid surfaces. Not all surfaces are smooth, though a bulk of the available information deals with smooth surfaces. Wavy irregular surfaces are commonly encountered in heat transfer devices which include solar collectors, regenerative heat exchangers and underground energy storage systems.
They have a significant role to play in the heat transfer processes in these applications. Since they bring in an added complexity due to the undulations in the surfaces, they need to be tackled with mathematical finesse through elegant simplification techniques. Also they do affect the flow and heat transfer characteristics, thereby behaving differently from straight smooth surfaces.
For a visual experience of natural convection, a glass filled with hot water and some red food dye may be placed inside a fish tank with cold, clear water. The convection currents of the red liquid may be seen to rise and fall in different regions, then eventually settle, illustrating the process as heat gradients are dissipated. Heat is produced in the body by the continuous metabolism of nutrients which provides energy for the systems of the body.
Therefore, excess heat must be dissipated from the body to keep it from overheating. When a person engages in elevated levels of physical activity, the body requires additional fuel which increases the metabolic rate and the rate of heat production. The body must then use additional methods to remove the additional heat produced in order to keep the internal temperature at a healthy level. Heat transfer by convection is driven by the movement of fluids over the surface of the body.
This convective fluid can be either a liquid or a gas. For heat transfer from the outer surface of the body, the convection mechanism is dependent on the surface area of the body, the velocity of the air, and the temperature gradient between the surface of the skin and the ambient air.
Convective heat transfer - Wikipedia
Heat transfer occurs more readily when the temperature of the surroundings is significantly less than the normal body temperature. Clothing can be considered an insulator which provides thermal resistance to heat flow over the covered portion of the body. This smaller temperature gradient between the surface temperature and the ambient temperature will cause a lower rate of heat transfer than if the skin were not covered. In order to ensure that one portion of the body is not significantly hotter than another portion, heat must be distributed evenly through the bodily tissues.
Blood flowing through blood vessels acts as a convective fluid and helps to prevent any buildup of excess heat inside the tissues of the body. This flow of blood through the vessels can be modeled as pipe flow in an engineering system. The heat carried by the blood is determined by the temperature of the surrounding tissue, the diameter of the blood vessel, the thickness of the fluid , velocity of the flow, and the heat transfer coefficient of the blood.
The velocity, blood vessel diameter, and the fluid thickness can all be related with the Reynolds Number , a dimensionless number used in fluid mechanics to characterize the flow of fluids.
Search Site Content
Latent heat loss, also known as evaporative heat loss, accounts for a large fraction of heat loss from the body. When the core temperature of the body increases, the body triggers sweat glands in the skin to bring additional moisture to the surface of the skin. The liquid is then transformed into vapor which removes heat from the surface of the body. The body continuously loses water by evaporation but the most significant amount of heat loss occurs during periods of increased physical activity.
Evaporative cooling happens when water vapor is added to the surrounding air.
- .
- ;
- ?
- History Repeating.
- The Circle of Love;
The energy needed to evaporate the water is taken from the air in the form of sensible heat and converted into latent heat, while the air remains at a constant enthalpy. Latent heat describes the amount of heat that is needed to evaporate the liquid; this heat comes from the liquid itself and the surrounding gas and surfaces.
Convective heat transfer
The greater the difference between the two temperatures, the greater the evaporative cooling effect. When the temperatures are the same, no net evaporation of water in air occurs; thus, there is no cooling effect. Magnetic evaporative cooling is a process for lowering the temperature of a group of atoms, after pre-cooled by methods such as laser cooling. Magnetic refrigeration cools below 0. Radiative cooling is the process by which a body loses heat by radiation.
Outgoing energy is an important effect in the Earth's energy budget. In the case of the Earth-atmosphere system, it refers to the process by which long-wave infrared radiation is emitted to balance the absorption of short-wave visible energy from the Sun. Convective transport of heat and evaporative transport of latent heat both remove heat from the surface and redistribute it in the atmosphere. Thermal energy storage includes technologies for collecting and storing energy for later use.
It may be employed to balance energy demand between day and nighttime. The thermal reservoir may be maintained at a temperature above or below that of the ambient environment. Applications include space heating, domestic or process hot water systems, or generating electricity. From Wikipedia, the free encyclopedia. Magnetic refrigeration and Magnetic evaporative cooling. Transport Processes and Separation Principles 4th ed.
Archived from the original on 10 December Retrieved 9 April IV; Lienhard, John H. A Heat Transfer Textbook 3rd ed. Fundamentals of momentum, heat, and mass transfer 2nd ed. Advanced Heat and Mass Transfer. International Communications in Heat and Mass Transfer. Introduction to Chemical Engineering Thermodynamics 7th ed.
- Convective Heat Transfer.
- ?
- Vous descendrez à larrêt Roussillon (French Edition);
- The Paper Bag Baby!
A practical approach 2nd ed. Fundamentals of heat and mass transfer 7th ed. Thermal Radiation Heat Transfer. Renewable and Sustainable Energy Reviews. Journal of Renewable and Sustainable Energy. Ryan Dupont and Kumar Ganesan Editors The Waste Management Approach to the 21st Century.
Section 27, page Upper Saddle River, New Jersey: When a substance condenses from a gas to a liquid, the same amount of heat is involved, but the heat is emitted rather than absorbed.
Heat transfer
Michael Hogan Sulfur , Encyclopedia of Earth, eds. Retrieved December 21, Van Wylen and R.
- Navigation menu?
- Japan, Korea and the 2002 World Cup.
- Heat Transfer?
- Browse Articles....
Often the term heat engine is used in a broader sense to include all devices that produce work, either through heat transfer or combustion, even though the device does not operate in a thermodynamic cycle. The internal-combustion engine and the gas turbine are examples of such devices, and calling these heat engines is an acceptable use of the term. Lytron Total Thermal Solutions. Retrieved 12 December Retrieved March 2, Physiology of Sport and Exercise 6th ed. Heating, ventilation and air conditioning. Architectural acoustics Architectural engineering Architectural technologist Building services engineering Building information modeling BIM Deep energy retrofit Duct leakage testing Environmental engineering Hydronic balancing Kitchen exhaust cleaning Mechanical engineering Mechanical, electrical, and plumbing Mold growth, assessment, and remediation Refrigerant reclamation Testing, adjusting, balancing.
History of chemical engineering. Unit operations Unit processes Chemical engineer Chemical process. Momentum transfer Heat transfer Mass transfer. Chemical reaction engineering Chemical kinetics Chemical process modeling. Process design Fluid dynamics Chemical plant design Chemical thermodynamics Transport phenomena.
